THE PHENOMENON OF PAIN AND MASSAGE THERAPY:
GATE-CONTROL THEORY OF PAIN
We continue to review how massage therapy heals the human body via various healing mechanisms we summarized in Fig 1. We initiated this discussion in the January/February (Part I) and March/April (Part II) 2010 issues of JMS, covering cellular stimulation, the phenomenon of piezoelectricity, and streaming potentials. We continued the discussion in Issues #3 and #4 of 2011, where we examined the effect of massage therapy on vasodilation. In this and the following articles, we will examine how massage therapy is able to control the pain analyzing system. Since the issue of pain is a complex subject, this article addresses only its key elements, focusing on the massage therapy perspective.
In Fig. 1 we summarized the clinical effects of massage therapy. We based this table on publications which have scientifically examined the therapeutic mechanisms of massage therapy.
Fig. 1. Local healing mechanisms of massage therapy
Pain as a phenomenon goes well beyond our traditional understanding of body functions in health or sickness. Besides being a physiological phenomenon, pain is also a philosophical concept that has fascinated humans throughout history.
In 1882, German philosopher F. Nietzsche who for years suffered from chronic pain, tried to use humor while grasping to describe his experiences (Bourke, 2013): “I have given a name to my pain. It is called ‘dog’ – just as faithful, just as obtrusive and shameless, just as entertaining, just as clever as any other dog, and I can scold it and vent my bad mood on it, as others do with their dogs and servants.”
Despite the incredible advances of medical science, pain and its control remain one of the cornerstones of modern medicine. For example, in Europe, 19% of its entire population lives with non-cancer chronic pain (Reid et. al., 2011) and this is an astonishing number. Thus, the matter of pain elimination or at least its control is a topic of great importance for physicians and patients, and manual therapy and medical massage play a huge role in this area.
In this and subsequent articles, we will discuss the modern understanding of pain science from the perspectives of manual therapy and medical massage. We think this is a critically important subject for practitioners. Without a clear understanding of how the pain analyzing system works the therapist relies on anecdotal experiences of teachers, co-workers and various ‘educators’ instead of modern science. In our clinic, we regularly observe the grim consequences of knowledge deficiency in this field by practitioners.
Pain is a guardian angel for humans, but at the same time, it is a pure devil when it gets out of control. Besides ruining the patient’s life, pain can become the biggest enemy the practitioner faces when trying to help a patient. Without efficient and quick control over the pain-analyzing system, all treatment protocols become a useless waste.
There are four basic practical concepts we would like the practitioner to remember in regard to pain and its control:
1. Pain isn’t a disease but a consequence of pathological abnormalities in the function of the somatic or visceral systems. Thus, don’t chase the pain; it is a ghost! Always find the original trigger!
2. In many clinical cases, the real cause of the pain isn’t where the patient indicated it is.
3. Pain control is a main treatment priority and must be achieved asap by any possible means. Only after this important task is accomplished by the therapist should the other issues (structural misbalance, postural changes, etc.) be addressed.
4. Pain has peripheral AND central components which need to be equally addressed.
Recently in the field of somatic rehabilitation, pain and measures of its control have become a very hot topic. The Internet is filled with blogs and webinars on this subject. Some of them provide excellent scientific data, while some ‘educators’ twist pain science and mislead the rest of the professional audience. We will address these issues as well.
A SHORT HISTORY OF PAIN SCIENCE
Since pain science is such a fundamental aspect of medicine, including somatic rehabilitation, we think it’s appropriate to start with a review of its short history.
We can trace the first scientific interest in pain to French philosopher René Descartes, who in the 17th century formulated the first theory of how pain is generated in our body and how we respond to it. Descartes’ views dominated the science of pain for over 300 years, and his ideas formed the so-called Specificity Theory of Pain. Specificity theory established the simple cause-and-effect logical chain: pain receptors were supposed to selectively detect noxious (i.e., harmful) stimuli and, through large and small fibers, transmit them directly to the brain via the spinal cord. The brain then activates an action system to form or change the body’s response. Terms such as noxious stimuli and nociception refer to harmful stimuli that ultimately lead to the formation of pain sensations in the brain.
Despite the fact that Specificity Theory dominated medicine for so long, it didn’t explain various pain phenomena associated with activation of the pain-analyzing system. The Specificity Theory couldn’t be right since it fails to explain why patients after an amputated limb continue for years to suffer from phantom pains. If the part of the limb with pain receptors is amputated, then according to the straight-through model of the pain system offered by Specificity Theory, the amputee is not supposed to feel any pain afterwards.
Up to the 1960s, various theories were proposed to explain the nature of pain, including the Summation Theory, Pattern Theory, and Sensory Interaction Theory, among others. All of them failed at one critical point. Their authors saw the brain itself as “…a passive receiver of messages” (Melzack and Katz, 2013). It took the work of two brilliant scientists, Prof. Ronald Melzack and Prof. Patrick Wall, who in 1965 published a journal Science article titled “Pain Mechanisms: A New Theory.” This article introduced the Gate Control Theory of Pain and it became the turning point in our understanding of pain and its mechanisms.
THE GATE CONTROL THEORY OF PAIN (GCT)
GCT is universally accepted as a basis of pain science by the international medical community. However, in the field of somatic rehabilitation, some ‘educators’ challenged and undermined the clinical value of GCT, greatly misleading practitioners. It occurred due to an incorrect understanding and misrepresentation of recent developments in the science of pain. The main argument of those who blindly followed pseudoscience is that Prof. R. Melzack developed a new theory, which he called the Neuromatrix Theory, and this new theory completely negates GCT. We will discuss Neuromatrix Theory in Part II of this article in the next issue of JMS.
While reading articles and monitoring Internet discussions on this subject, we realized that authors and practitioners who twisted science didn’t fully understand GCT and its enormous clinical impact. This is why we felt obligated to explain to practitioners the nature and practical value of GCT in a step-by-step discussion. At first glance, the information presented below may appear complex, but it is as simple an explanation as possible, and it’s worth reviewing carefully to fully grasp the concept. Understanding GCT has a profound impact in the therapy room, and we will discuss this issue further in the following sections of this article.
First, let’s examine the anatomophysiological arrangement of the basic components of the pain analysis system according to GCT. They are: Peripheral Receptors, Sensory Pathways, Substantia Gelatinosa, T-cells and Central Control Trigger. Fig. 2 shows the basic arrangement of the pain analyzing system, where these components are indicated.
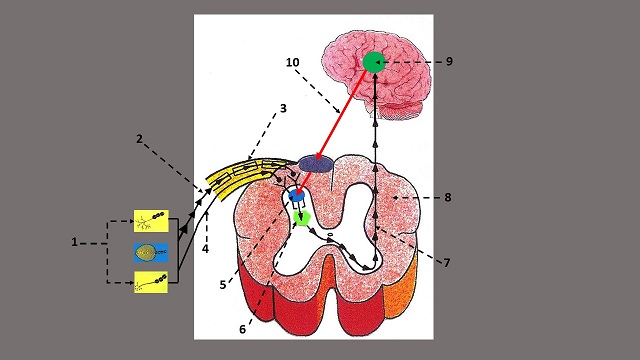
Fig. 2. Anatomical arrangement of the components of the Gate Control System
| 1 – peripheral receptors in the soft tissues 2 – sensory part of the spinal nerve 3 – A (myelinated) small fibers 4 – C (unmyelinated) large fibers 5 – Substantia Gelatinosa with gates |
6 – T-cells 7 – ascending sensory flow from T-cells 8 – cross section of the spinal cord 9 – hypothalamus 10 – system of Central Control Trigger |
Peripheral Receptors (PR)
The first step in pain formation is activation of peripheral receptors, including nociceptors (a.k.a. pain receptors) located in the soft tissues (see Fig. 2 ‘1’), which form an ascending flow of sensory information from the soft tissue to the CNS.
This activation happens as initial noxious stimuli exceed the threshold of peripheral receptor activation. The activation of peripheral receptors results from changes in ion channels within each receptor (Shipton, 2013). As soon as the ion channels mediate the transfer of physical (e.g. mechanical pressure) or chemical (exposure to a strong chemical irritant) stimuli into the sensory stimuli, they start to travel to the spinal cord and into the higher brain centers where pain perception is finalized.
If the patient suffers from chronic pain the threshold of activation of all peripheral receptors located in the affected area significantly drops (Gangadharan and Kuner, 2013) and the condition of hyperirritability of peripheral receptors is developed. In such a case, all receptors start to fire to the CNS earlier than they should, overwhelming the CNS and enhancing pain perception through the additional summation of stimuli.
Some peripheral receptors can act as purely sensory receptors, while others can function as both sensory and nociceptors (i.e., receptors that detect harmful stimuli) simultaneously. For example, the application of pressure in the soft tissues produces the sensation of pressure (i.e., pure sensory stimuli), but a further increase in pressure triggers pain perception in the same area (i.e., noxious stimulation). Another example is temperature receptors, which can detect fluctuation of temperature (i.e., purely sensory stimuli) or trigger pain perception if the temperature increases or decreases above or below the normal threshold (i.e., noxious stimulation).
Somatic practitioners who challenge the notion of the peripheral origin of pain also deny the existence of special peripheral receptors that act solely as nociceptors or pain receptors. In reality, our body has so-called free (sensory) nerve endings which act as peripheral receptors for pain. It is difficult to believe but while scientists around the world (Moraes et al., 2011: Rein et al., 2013; Witt and Vilensky, 2014, etc.) study free nerve endings and their role in pain formation, some ‘educators’ and somatic and massage practitioners deny even the fact of their existence! Here is a quote from a recent scientific article (Gregory and Sluka, 2014):
“Muscle pain is mediated by free nerve endings (bold by JMS) distributed through the muscle along arteries… Mechanical forces, ischemia, and inflammation are the primary stimuli for muscle pain, which is reflected in the array of peripheral receptors contributing to muscle pain… Sensitization of peripheral receptors and of central pain processing structures are both critical for the development and maintenance of chronic muscle pain.”
Sensory Pathways (SP)
The sensory signals generated in the peripheral receptors arrive at the spinal cord via small myelinated or A fibers (see Fig. 2, ‘2’) and large unmyelinated or C fibers (see Fig. 2, ‘4’) which are together within the same sensory part of the spinal nerve (Fig. 2, ‘3`).
Having a myelin sheath around the nerve greatly increases the speed of nervous impulse conduction. One can make an analogy between a highway (A fibers) and a dirt road (C fibers). In both scenarios, the driver will get from point A to point B, but he or she will do it more quickly using the highway (A fibers) compared to the dirt road (C fibers).
A and C fibers work with their own group of peripheral receptors in the soft tissues, which generate sensory signals independently. It helps to more efficiently control any changes in the outside or inside environment, including nociception. The critical factor in the formation of pain perception and its control is the balance between A- and C-fibers. We will discuss this important subject in the following section.
A and C fibers end at the dorsal horns of the spinal cord, which work as a sensory computer, primarily analyzing sensory input. The first element of this system is special cells called the Substantia Gelatinosa.
Substantia Gelatinosa (SG)
SG is a layer of tightly packed cells in the dorsal horns of the spinal cord (see Fig. 2, ‘5’). All sensory information that arrives from the peripheral receptors to the CNS activates or inhibits SG first, and these cells modulate sensory input and transfer it further to T-cells (i.e., transmission cells).
A-fibers stimulate SG, and in contrast, C-fibers inhibit the activity of SG. As a result, the activity of T-cells can be altered in the same way. Thus, besides transferring function, SG acts as a ‘gatekeeper’ since these cells may additionally suppress or enhance the initial sensory input that arrives at the CNS from peripheral receptors. Thus, SG controls how much stimulus is transferred to the next stage, which is the T-cells.
Transmission Cells (T-cells)
The next stage in nociception is activation of T-cells (see Fig. 2, ‘6’). Nociceptive information from SG is now being transferred to T-cells, which project it centrally, i.e., to the brain. If nociceptive stimuli arrive via the highway (A-fibers), SG additionally enhances these stimuli through their summation. Such excessive stimulation of T-cells exceeds their threshold, and they become the first component in the formation of pain perception by the brain and the body’s response to any type of noxious stimulation.
The intimate relationships between SG and T-cells explain why we experience pain even after the noxious stimulation has stopped. Once SGs are activated by initial noxious stimuli, these cells remain active for some time, continuously stimulating T-cells even after the noxious stimuli are withdrawn. In such a case, T-cells continued to send sensory information to the brain, where pain perception is formed (see Fig. 2, ‘7-9’).
Central Control Trigger (CCT)
Like others, Prof. R. Melzack and Prof. P. Wall were well aware of countless reports of badly wounded people acting as if their injury didn’t happen (e.g., a badly wounded soldier continues to run on the battlefield). Thus, our emotions, fears, and anxieties significantly impact our perception of pain.
Additionally, a very interesting experiment was conducted by Prof. I.P. Palvov in the 1920s, which has puzzled pain scientists ever since. When Pavlov’s dogs’ skin was electrically shocked or cut, the animals behaved as if they were in pain. However, if food was immediately given after noxious stimulation, to Prof. Pavlov’s surprise, the dogs eventually stopped reacting to the noxious stimulation over time. Instead, they started to salivate and produce gastric juice in expectation of food.
These and other examples must have their explanation, so the fathers of GCT concluded there is supposed to be some central (i.e., in the brain) mechanism that is able to modulate or alter noxious stimulation arriving at the spinal cord, and this phenomenon will be able to affect the formation of pain perception by the brain. They called this mechanism the Central Control Trigger.
Prof. Melzack and Prof. Wall’s theory was correct because the brain indeed is able to elicit descending control over the gates located in the segments of the spinal cord and assist SG to keep the gates in a complete or partly closed position, despite that significant trauma has occurred to the soft tissues and overwhelming noxious input is present (see Fig. 2, ’10’).
THE PHYSIOLOGICAL CHAIN OF EVENTS WITHIN THE GATE-CONTROL SYSTEM
Let’s examine the physiology of the Gate Control System from a somatic rehabilitation perspective to make pain science more relatable to readers. To better illustrate the complex process of pain formation and simplify it as much as possible, we will use Figs. 3 and 4. In both figures, SG stands for Substantia Gelatinosa. The plus symbol means stimulation. The minus symbol means inhibition. The red and yellow sparks are sensory stimuli that travel from the peripheral receptors to and within the CNS.
Fig. 3. The Gate Control System when inappropriate pressure is used during bodywork
At the outset, I would like to remind readers that, as mentioned above, the critical factor in the formation of pain perception and its control is the balance between A (small, fast) and C (large, slow) fibers. Disproportionate activation of A-fibers will greatly stimulate the pain analyzing system, while activation of C-fibers will inhibit its activity.
Let’s assume that during deep tissue massage, the therapist uses an inappropriate degree of pressure, centering his or her therapy on the ‘no pain, no gain’ concept. By doing that, the therapist immediately recruits a larger number of peripheral receptors in the soft tissues, and as a result, sensory signals will arrive at the SG via the highway of A-fibers (Fig. 3: train of red sparks).
When sensory stimuli arrive at the Substantia Gelatinosa (Fig. 3: SG) via A-fibers, they inhibit the SG (Fig. 3: first red sphere with minus symbol below SG). If the SG is suppressed, it is unable (Fig. 3: large red ‘X’ symbol) to elicit its own inhibitory control (Fig. 3: two yellow spheres with minus symbol) over both A- and C-fibers before they come in contact with T-cells. As a result, gates are open, noxious stimuli are pouring uncontrolled into the T-cells via A-fibers, and from there into the brain. Thus, A-fibers, when unopposed by the SG, stimulate the T-cells (Fig. 3: second red sphere with plus symbol, below T-cells). Activation of T-cells causes the summation of noxious stimuli and the formation of a sensory flow of noxious information to the brain, more specifically to the hypothalamus.
The formation of pain reception by the brain activates an action system that is now in full alert mode. This means the patient tries to withdraw the body or its segment from the therapist, tenses all muscles in the treated area or the entire body, holds their breath, forms a negative perception of the therapist and treatment, etc. These and other destructive outcomes negate the clinical potential of the treatment in the long run, despite the fact that immediately after such ‘therapy’, the patient may feel better for the simple reason that the ‘torture’ has stopped.
As mentioned above, one of the first outcomes of somatic abnormalities is a significant decrease in the threshold of activation for peripheral receptors. In such a case, even mild stimuli become noxious. This is why the therapist must work around the patient’s pain threshold but has to do it in a defensive way (just below pain threshold) to have as much control as possible over the patient’s pain analyzing system. This is the first critical step in successful somatic rehabilitation.
Now, let’s assume the therapist starts to work on the soft tissues in the affected area using various clinical tools to control the pain analyzing system BEFORE he or she intends to activate it. Fig. 4 illustrates the chain of events within the Gate Control System in these cases.
Fig. 4. The Gate Control System when the pain-analyzing system is under control during the session
If the therapist starts treatment without using any noxious stimuli and applies the correct tools of pain control, they engage a smaller number of peripheral receptors, and the sensory information will be conducted via unmyelinated C-fibers (Fig. 4: yellow sparks). These stimuli will reach and activate the SG first (Fig. 4: first yellow sphere with plus symbol, above SG). An activated SG will inhibit stimulation of T-cells by suppressing the activity of both the A- and C-fibers before they come into contact with T-cells (Fig. 4: two yellow spheres with minus symbol). In such a case, the SG partly or completely closes the gates, preventing T-cells from firing to the brain in response to any noxious stimuli. If, while the gates are closed, the practitioner carefully applies noxious stimuli (if needed for the therapy), they can’t or can only partly reach T-cells, as the SG blocks their advance via A- or C-fibers (Fig. 4: two large yellow ‘X’ symbols).
An additional element the therapist must use as a tool to control the pain analyzing system is the System of Central Control Trigger (CCT). As we discussed above, our attention, emotional state, fears, and hopes alter the way nociceptive stimuli are delivered through the CNS, and subsequently, it affects pain perception formed by the brain. If intense nociception is triggered and sensory information arrives via A-fibers, the CCT doesn’t have time to be employed. As Prof. Melzack and Prof. Wall (1965) stated:
“Some of the most unbearable pains, such as cardiac pain, rise so rapidly in intensity that the patient is unable to achieve any control over them (i.e., via CCT by JMS).
However, when the therapist works defensively without exceeding the pain threshold and activates the central control trigger BEFORE applied pressure reaches the pain threshold level, he or she will be able to employ the inhibiting function of CCT. CCT, together with C-fibers, is able to stimulate the SG (Fig. 4, white sphere with plus symbol), and it assists in keeping gates in the closed position.
In all cases of bodywork that aim for clinical outcomes through massage therapy, the therapist must work just below the pain threshold. If Trigger Point Therapy is used, the therapist works on the level of pain threshold (first minimal sensation of pain). In these cases, the control of the pain analyzing system becomes a critical factor. If the therapist employs correct clinical tools, such as an inhibitory regime of massage therapy and electric vibration, they artificially raise the background activity of C-fibers, which allows for the suppression of A-fiber activity, and the therapist gains better control over the entire pain analysis system (Melzak and Wall, 1965).
At this point, we have discussed how sensory nociception reaches the brain, where pain perception is formed. In this article, we will stop here. What will happen next in the brain itself, we will discuss in Part 2 of this article in issue #4 of JMS.
It is rarely mentioned but in their initial article (1965) about the Gate-Control Theory of Pain, Prof. Melzack and Prof. Wall clearly stated the importance of bodywork as a tool to control pain:
“Conversely, any manipulation that cuts down the sensory input lessens the opportunity for summation and pain, within the functional limits set by the opposing roles of the large (C) and small (A) fiber systems.”
Here are following manipulations which are mentioned in the article: massage, light percussion, vibration, and gently moving water. However, when a therapist works with the completely inappropriate and unprofessional mindset of “no pain, no gain,” he or she actively fights with the body of the patient and he or she will lose this fight in the long run because the patient’s defense system is much smarter and efficient than the therapist’s wrong set of beliefs.
THE CLINICAL SIGNIFICANCE OF THE GATE CONTROL THEORY OF PAIN FOR MODERN MEDICINE
In the late 1990s, Prof. Melzack developed a new pain theory, which he called the Neuromatrix Theory (NT). All of his and his co-author, Prof. J. Katz’s, articles on this subject emphasized that NT is a further development of the same theoretical concept, originating from the GCT.
As we mentioned above, some chiropractors, physical therapists and massage practitioners concluded, based on incorrect reading of the articles or on incorrect presentations by some ‘educators,’ that GCT was centered on the notion of the peripheral origin of pain while new NT developed by Prof. Melzack completely denied GCT and states that pain has only a central origin.
Unfortunately, these unscientific views spread rapidly among somatic practitioners, further diminishing their clinical effectiveness. To warn those who had not yet been infected with such absurd notions, we asked Prof. Melzack and Prof. Katz for an interview to clarify the connections between the two theories and the unprecedented impact GCT still has on modern medicine. Both scientists were courteous enough to answer our questions, and we published the interview and our follow-up article in Issue #4 of JMS, 2013.
The publication of these materials generated very unexpected results. To our great surprise, in several professional Internet blogs, practitioners accused JMS of fabricating the entire interview, or at least of misleading two world authorities in pain science with our questions. It seems that Prof. Melzack and Prof. Katz’s interview suddenly removed ground from under the pseudoscience that recently flourished in the somatic rehabilitation field regarding pain and its control.
How did it happen that the GCT, which is one of the cornerstones of modern medicine, became a ‘wrong and outdated theory’ among some somatic practitioners? The inability of some practitioners to analyze a scientific paper published in 1965, the blind following of ‘educators’ who twisted science for personal or monetary gain, and blaming the patient’s brain as a reason for failed treatment protocols – these and other factors undermined the value of GCT in their eyes.
In reality, the critical mistake these practitioners made is not understanding that GCT, from the very beginning, emphasized both the peripheral and central origins of pain. If you carefully read this article, which is based on Prof. Melzack and Prof. Wall’s original publication, you will now see how important the central origin of pain is to the GCT. In fact, the GCT was the first step in our understanding of the role the brain plays in the formation of pain perception, and this was one of its greatest achievements.
Here is what Prof. Melzack and Prof. Katz the authors of Neuromatrix Theory said (2013) on the value of GCT:
“This (new data about phantom pain, by JMS) does not negate the gate theory (bold by JMS), of course. Peripheral and spinal processes are obviously an important part of pain, and we need to know more about the mechanisms of peripheral inflammation, spinal modulation, midbrain descending control, and so forth.”
Here is a quote from the British Journal of Anaesthesia (2002) which published a special editorial article, “Gate Control Theory of Pain Stands Test of Time,” long after the initial publication of Neutomatrix Theory:
“GCT stated in an elegant and succinct way that the transmission of pain from the peripheral nerve through the spinal cord was subject to modulation by both intrinsic neurons and controls emanating from the brain.”
It is unfortunate to see that those who criticized the GCT as an outdated concept still use its fruits every time they or their relatives undergo surgery under general anesthesia, while denying GCT for their patients in the therapy room.
Since somatic practitioners work mostly with the musculoskeletal system, we would like to cite one more quote from the article, “Peripheral and Central Mechanisms of Chronic Musculoskeltal Pain” by Professor A. Sluka (2013):
“Chronic musculoskeltal pain clearly involves activation of both peripheral and central pathways. In some patients the pain is primarily maintained by peripheral inputs while in others the pain is maintained primarily by central changes; however, the majority of patients probably have a combination of both (bold by JMS) peripheral and central changes”
In Part II of this article, which will be published in the next issue of JMS, we will discuss the Neuromatrix Theory of pain from a somatic rehabilitation perspective.
Bourke J. What is pain? A history of the Prothero lecture. Trans R Hist Soc. 2013 Dec: 23:155-173
Carr F.B., Zachariou V. Nociception and pain: lessons from optogenetics. Front Behav Neurosci. 2014 Mar 25; 8:69
Gangadharan V. and Kuner, R. Pain Hypersensitivity Mechanisms at a Glance. Dis. Model. Mech. Jul 2013: 6(4): 889-895
Gregory NS, Sluka KA. Anatomical and Physiological Factors Contributing to Chronic Muscle Pain. Curr Top Behav Neurosci. 2014
Melzack R., Katz J. Pain. Wiley Interdisciplinary Reviews: Cognitive Science. Jan/Feb. 2013, 4(1):1-15
Melzack R., Wall P.D. Pain Mechanisms: A New Theory. Science. Nov. 1965, 150(3699): 971-979
Moraes MR, Cavalcante ML, Leite JA, Macedo JN, Sampaio ML, Jamacaru VF, Santana MG. The characteristics of the mechanoreceptors of the hip with arthrosis. J Orthop Surg Res. 2011 Nov 16;6:58
Pavlov I.P. Lectures on Conditioned Reflexes. International Publishers, New York, 1928
Reid K.J., Harker J., Bala M. M., Truyers C., Kellen E., Bekkering G. E., Kleijnen J. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr. Res. Med. Opin. 27; 449-462
Rein S, Hanisch U, Zwipp H, Fieguth A, Lwowski S, Hagert E. Comparative analysis of inter- and intraligamentous distribution of sensory nerve endings in ankle ligaments: a cadaver study. Foot Ankle Int. 2013 Jul;34(7):1017-24
Shipton E.A. Skin Matters: Identifying Pain Mechanisms and Predicting Treatment Outcomes. Neurol Res Int. 2013: 329-364
Sluka K.A. Peripheral and Central Mechanisms of Chronic Musculoskeletal Pain. Pain Management. 2013, Mar 1; 3(2): 103-107
Witt KL, Vilensky JA. The anatomy of osteoarthritic joint pain. Clin Anat. 2014 Apr;27(3):451-4
Category: Medical Massage
