HOW MASSAGE HEALS THE BODY. PART V
STIMULATION OF CELLULAR FUNCTIONS BY MASSAGE THERAPY
By Dr. Ross Turchaninov
We continue to publish articles which review various mechanisms responsible for the clinical effects of Massage Therapy (MT). These articles are united by a common theme: How Massage Heals the Body. If you haven’t read the previous articles yet, please do so since they will equip you with priceless data that will help you understand what you are actually doing while applying MT.
Another important aspect of this data is that it will help you build bridges with other health professions. If you want to establish a solid referral base you have to speak with physicians, chiropractors or physical therapists using the same language and concepts. These articles give you this ability. Readers may find previous articles which cover other aspects of MT’s healing impact here:
Part I. Piezoelectrical Phenomenon in the Soft Tissues. JMS Issue #1, 2010
Part II. Streaming Potentials: JMS Issue #2, 2010
Part III. Vasodilation Mechanisms: JMS Issue #3, and Issue #4, 2011
Part IV. Phenomenon of Pain and Massage Therapy: Gate-Control Theory of the Pain and MT: JMS Issue #3, 2014
Neuromatrix Theory of the Pain and MT: JMS Issue #4, 2014
Clinical Application of Gate-Control and Neuromatrix Theories of the Pain: JMS Issue #1, 2015
This article addresses the fact that MT deeply impacts cellular function and if therapists understand events which are triggered by MT even on the cellular level they will be able to optimize treatment and better help patients.
We think everyone agrees that MT is a way to deliver mechanical force to the soft tissues and the pressure itself is a healing factor. From this perspective, medical science has accumulated a lot of data which illustrate profound changes in the function of the cells when they function under the application of direct pressure. However, to fully understand and appreciate this data, which has great practical value for therapists, we need to quickly review some very basic principles of cellular biology.
CELLULAR BIOLOGY
Every cell is composed from several main components: cellular membrane and cytoskeleton, cytoplasm with organelles, and nucleus with nucleus (see Fig. 1).
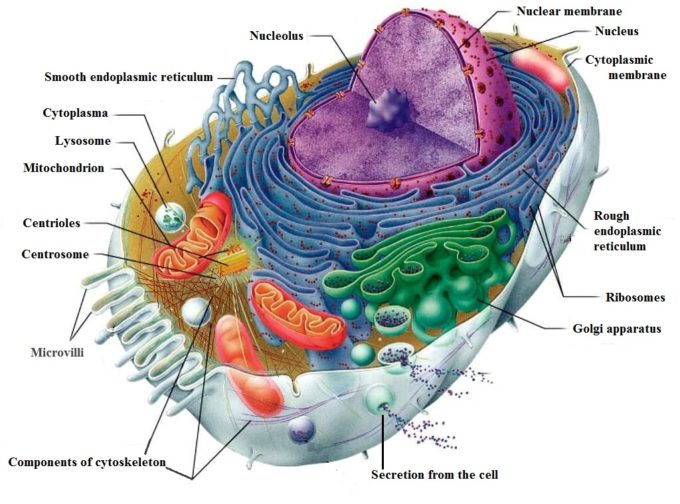
Fig. 1. Structure of the Cell
The structure and functions of all cellular components are well known and it is not the subject of our discussion. However, findings about the cytoskeleton and its relation to external mechanical force applied to the cell are breakthroughs in the understanding of the therapeutic effect of the mechano-stimulation including MT of the living cells.
The cytoskeleton is a complex system of fibrillar structures located in the cytoplasm and it connects cytoplasmic membrane to the nucleus. It can be compared to the human skeleton which provides a frame for our body and its dynamic support. Thus, the cytoskeleton gives the cell its form. For example, fibroblast (see Fig. 4), which is a major repair cell of the body has shape because the fibrils of the cytoskeleton are arranged in this particular way. Fig. 2 presents an electronic microphotograph of cytoskeleton of human keratinocyte (the most abundant cell in our epidermis). Notice that fibrils of cytoskeleton run between the cytoplasmic membrane and nuclear membrane.
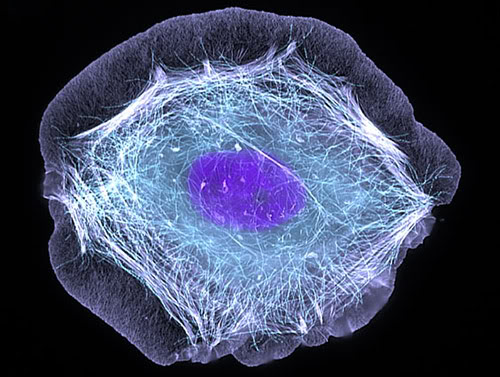
Fig. 2. Cytoskeleton of keratinocyte
In contrast with the body skeleton, which is a rigid structure, the cytoskeleton of the cell is very dynamic. It constantly moves since it must traffic organelles around the inner cell, excrete waste and bring nutrition in. The cytoskeleton can very quickly completely re-synthesize and renew itself and it consists of 3 types of fibrils: mictotubules, actin filaments and intermediate filaments.
From the time of its discovery at the beginning of the 20th century, the cytoskeleton was always viewed as a purely mechanical structure that provides the shape of the cell and participates in cell motility, migration (e.g., leukocytes, natural killer cells, etc.) and it is the key component of cell division.
However, modern experimental equipment allowed scientists to set up and conduct more detailed studies of the cytoskeleton. Thanks to these studies, we now know more important functions of the cytoskeleton:
- Controls functions of the cytoplasmic membrane which surrounds each cell.
- Connects the cytoplasmic membrane to the nucleus.
- Regulation of cellular fucntions: synthesis of proteins, hormones, enzymes and other substances which are generated within the cells and secreted from the cell.
- Indirectly controls DNA and individual genes’ expression (i.e., function).
Of course, we are interested in all these scientific data from the MT perspective since bodywork delivers the external mechanical stimulation to the soft tissues.
Control Over Cytoplasmic Membrane
Therapists work in the soft tissues with repetitive application of strokes which deliver mechanical force with fluctuating pressure. Every time the soft tissues are compressed, the pressure between cells, which compose the different layers of the soft tissue, also increases and fluctuates. These fluctuations are detected by the cytoplasmic membrane of each individual cell and they stimulate cell function.
A cytoplasmic membrane surrounds each cell and via this membrane the cells actively interact with the environment. It has a thickness of 7.5 nm, or l/4000 of an inch, and is made up of double layers of phospholipids with integrated proteins in it. Fig. 3 shows the structure of the cytoplasmic membrane.
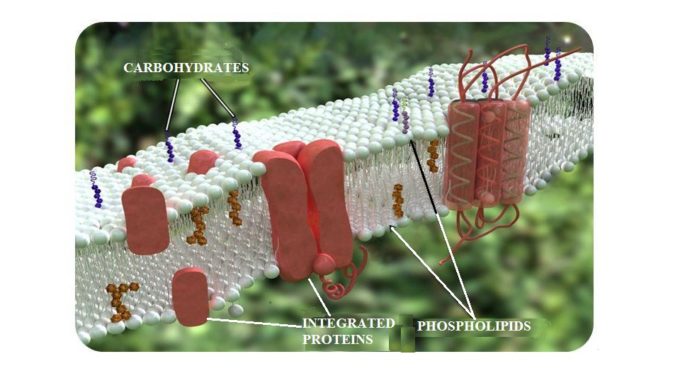
Fig. 3. Structure of cytoplasmic membrane
The integrated proteins which are imbedded into the cytoplasmic membrane work as gates that connect the external environment and inner part of the cell. Any molecules that the cell needs for its normal functions pass into the cell through these gates. They also allow the new synthesized proteins or waste to be secreted or removed from the cell.
The cytoplasmic proteins occupy approximately 50% of the membrane’s mass and form a net of receptors over its entire outer surface. Thus, any outside molecule must first interact with these receptors to be recognized. Only after this occurs will the gate open and let the molecule in.
Mechanical stimuli (e. g., in the form of massage strokes) affect the protein receptors located on the cytoplasmic membrane of all cells which compose the soft tissue therapists work with. When activated these receptors immediately convert mechanical energy applied from outside (e.g., by MT strokes) into chemical stimuli that conduct inside of the cell, stimulating or inhibiting its functions (Jain, et al., 1990; Komuro, et al., 1991).
Not all receptors in the cytoplasmic membrane act as mechanoreceptors. One particular family of proteins, called integrins, reacts to the mechanical stimulation (Wang, et al., 1993). Thus, for our discussion the integrins are extremely important and we will trace the events in the cells starting with the activation of those receptors.
Since the fibrils of the cytoskeleton are deeply anchored in the cytoplasmic membrane, the cytoskeleton controls function, arrangement and even number of mechanoreceptors which are working in the membrane. Thus, by regulation of number and function of cytoplasmic receptors, the cytoskeleton manages the metabolism of the whole cell (Gataullin and Zaripov, 1988; Jain, et al., 1990).
Connection of the Cytoplasmic Membrane to the Nucleoskeleton
Now let’s see how mechanical stimuli applied to the soft tissue and individual cells can alter the most sacred part of life: function of cell nucleus. As we know nucleus stores the vital information of all living objects – DNA. The nucleus is surrounded by its own nuclear membrane, which has pores that open into the cytoplasm and into the cellular structure called endoplasmic reticulum (see Fig. 2). Endoplasmic reticulum is part of the cell where everything the cell produces (proteins, hormones, enzymes, etc.) are synthesized before it is unloaded from the cell. The nucleus also has its own nucleoskeleton which has striking similarity with the cell’s cytoskeleton (McKeon et al., 1986).
One of the most important functions of the cytoskeleton is its ability to conduct information generated by the mechanical stimulation of receptors in cytoplasmic membrane directly into the nucleus (Maniotis et al., 1977). Such mechanical stimulation has an effect on the cell growth, its life span, function and even individual gene’s expression (Wang et al., 1993).
Control of DNA and Regulation of Cellular Function
Let’s look at the fascinating process of MT stimulating the cellular function using the example of the human fibroblast which undergoes repetitive mechanical stimulation during cross-fiber friction. Fig. 4 shows the human fibroblast (FB) under an electronic microscope.
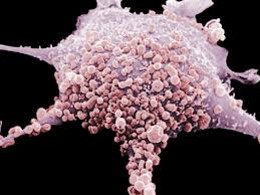
Fig. 4. Microphotograph of human fibroblast (FB)
FBs are therapists’ best friends and allies in the patient’s body. To correctly use and activate this major healing force, therapists must know what fibroblasts do and how they operate.
To deal with the consequences of any trauma and inflammation in the soft tissue or inner organs, the body employs FBs. They are everywhere and they will have concentrated in the injured or inflamed area to restore functions.
The FBs participate in healing by producing a special protein called pro-collagen. The genetic code for the synthesis of pro-collagen is stored within the nucleus of each FB. After pro-collagen synthesizes within FB it is unloaded from the cell into the extracellular matrix and it matures there into the collagen fibers which the body uses to heal injured or inflamed tissues or organs. A scar on your finger after you cut it with a knife, healing of the tendinitis, filling the damaged part of the brain after a stroke, etc. – all of that is the result of FBs producing and depositing pro-collagen.
The FBs are attracted to the injured or inflamed areas by the histamine released from the affected tissues as a result of their damage. However, when FBs arrive to the damaged or inflamed areas the mechanical pressure on the cytoplasmic membrane of the FB becomes one of the major driving forces for the stimulation of pro-collagen synthesis inside of FBs. This mechanical pressure comes from the edema in the affected tissues, but it can be enhanced by MT which stimulates function of FBs by additionally increasing pressure within the tissue.
Fig. 5 summarizes data from various scientific sources on how mechanical stimulation of the soft tissues in the form of MT activates a major healing force in the body – FBs. Please also refer to Fig. 1 for any additional info.
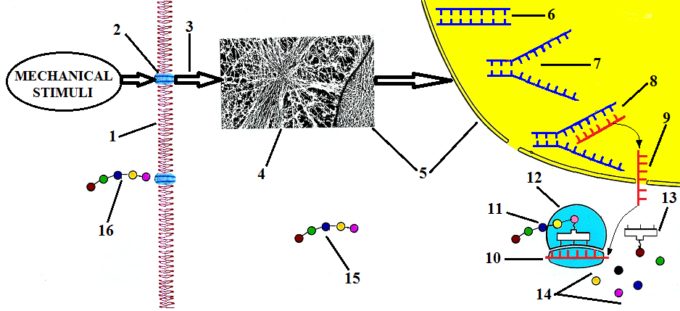
Fig. 5. Stimulation of FBs’ function by direct mechanical stimulation (cross-fiber friction)
The external mechanical stimuli in the form of repetitive friction additionally increase interstitial pressure and this increase is immediately detected by integrin receptors (Fig. 5: ‘2’) located in the cytoplasmic membrane (Fig. 5: ‘1’) of the FB. Integrin receptors convert mechanical stimuli applied to the membrane into chemical signals (Fig. 5: ‘3’). These signals trigger the reorientation of the cytoskeleton’s fibers (Fig. 5: ‘4’) in the direction of the applied force by the therapist.
The reorientation of cytoskeleton fibers increases pressure inside the cell in the direction of applied force and since the cytoskeleton is connected to the cell’s nucleus (Fig. 5: ‘5’) it causes its distortion. Inside of the nucleus the coil of DNA is stored (Fig. 5: ‘6’) and it is where the code about sequencing of pro-collagen is located.
Increased pressure inside of the nucleus and its distortion is a major trigger for the activation of DNA synthesis and its replication (Curtis and Seehar, 1978; Somjen et al., 1980; Brunett, 1984). The replication of DNA (Fig. 5: ‘7’) is split of its double coil in two identical sequences.
As soon as DNA split, the so-called Messenger-RNA or M-RNA (Fig. 5: ‘8’) starts to be synthesized on the available arm of DNA, reading the genetic code which contains the correct sequence of pro-collagen. As soon as M-RNA finishes reading the DNA sequence it now carries correct data about pro-collagen out the nucleus, leaving it via pores in the nuclear membrane (Fig. 5: ‘9’). These pores open directly into Rough Endoplasmic Reticulum (see Fig. 1). Here m-RNA associates with ribosomes (Fig. 5: ‘12’) which are pro-collagen synthesizing stations.
After newly synthesized M-RNA attaches itself to each ribosome (Fig. 5: ‘10’) so called Transport-RNA or T-RNA (Fig. 5: ‘13’) starts to catch individual amino acids (Fig. 5: ‘14’) which are floating within the Rough Endoplasmic Reticulum and transport each amino acid to the ribosome/M-RNA complex. Only those T-RNA which brought ‘correct’ amino acid to the sequence are allowed to attach to the newly synthesized molecule of pro-collagen (Fig. 5: ‘11’).
After a molecule of pro-collagen is fully synthesized in the Rough Endoplasmic Reticulum it travels through the cytoplasm (Fig. 5: ‘15’) until it reaches the cytoplasmic membrane where it secreted out of the FB into extracellular matrix (Fig. 5: ‘16’). After pro-collagen is unloaded from the FB it matures in the collagen fibers which the body uses to heal injured or inflamed tissues and organs.
Thus, mechanical stimuli activate the FBs and stimulate pro-collagen synthesis, which leads to the increasing of collagen and extracellular matrix production at the injured site. Collagen is the most abundant protein of any life organism and it comprises approximately 1/3 of all proteins in the body (Geneser, 1986).
Here are scientific data which support this critically important clinical effect of MT:
- Direct mechanical pressure on human fibroblasts causes cellular growth to increase 1.7 times, increasing protein synthesis up to 48%, in 24 hours after the mechanical stimulation (Jain et al., 1990).
- Davidson et al., (1997) examined the effect of soft tissue mobilization (intense cross-friction) on experimental tendinitis caused by the injection of enzyme collagenase into the Achilles tendon of 20 rats. The results obtained from experimental and control groups were examined with light microscopy, electron microscopy, immunoelectron microscopy and by gait analysis.
The authors found that the mean increase of the FB count in an experimental group was to 15 + 11, versus 3 + 3 in the control group. All FBs in the experimental group (with cross fiber friction) exhibited a highly developed rough endoplasmic reticulum, which is clear evidence of the stimulation of collagen production. Thus, the mechanical stimuli, cross-friction in this case, stimulate FBs which are working locally and attract the neighboring FBs to the site of inflammation. The clinical outcome of this stimulation is increase in collagen production and healing of affected tendons.
- Gehlsen et al., (1999) also examined the effect of soft tissue mobilization with different amounts of applied pressure on the FBs count. The authors used the same experimental model of tendinitis in the Achilles tendon. They found that the application of strong pressure stimulated the healing process in the tendon much faster (FB count = 375) compared to the treatment with light (FB count = 190) or moderate (FB count = 250) pressure.
For the first time in modem literature, the authors of this study established a clear connection between mechanical stimuli, their therapeutic effect on somatic pathology in the form of soft tissue mobilization, and their effect on cellular functions.
REVIEW OF OTHER SCIENTIFIC DATA
Events we described above are not limited to the stimulation of the FBs’ function only. Direct mechanical stimulation of the soft tissue has profound impact on other structural components of the body. Here are some examples:
- Mechanical pressure stimulates the new capillaries formation. This is especially important in the injured areas where capillaries were damaged by traumatic impact. Endothelial cells formed walls of capillaries and purely mechanical pressure stimulated their growth and correct arrangement.
In 1989 a very interesting study (Shirinsky et al., 1989) confirmed this fact. The authors isolated human endothelial cells in vitro, i.e., in the Petri dish, free from any hormonal or nervous system influence, and applied the mechanical pressure on the cell culture deforming the cytoplasmic membranes. The authors registered the rapid growth and elongation of endothelial cells within 3 hours (!) of applied pressure. However, the most astonishing phenomenon the authors registered in 48 hours after the mechanical stimulation of the endothelial cells – the culture of the cells became uniformly oriented along the axis of initial application of pressure which coincided with the future orientation of the new capillary. All of that happened in the Petri dish just after mechanical stimulation of the endothelial cells!
This information has great practical value since if therapists don’t apply pressure in the correct direction and under the correct angle, MT won’t be able to stimulate formation of the new capillary network in the damaged soft tissues.
- It is a commonly accepted fact that passive stretching helps a muscle to relax. This is completely true if the muscle carries tension or spasm. However, passive stretching of the skeletal muscle within physiological range greatly stimulates healthy muscle performance.
Chen and Grinnel in a 1995 experimental study found that stretching the skeletal muscle of a frog in the physiological range (up to 2 mm) more than doubled the spontaneous and evoked release of the neurotransmitter acethylcholine from the motor nerve terminal. Acetylcholine is used in the neuromuscular junctions as a neurotransmitter which is responsible for the transfer of electric impulses from the nerve to the muscle the nerve innervates.
The authors concluded that increase in the pressure in the skeletal muscle during the passive stretch activates integrin receptors in the cytoplasmic membrane. Additionally, it mobilizes Ca+2 which assists in the release of extra acetylcholine. More acetylcholine is available in the neuromuscular junction’s more responsive muscle to the motor commands from the CNS.
This study validated the fact that light stretching of the skeletal muscles at the end of massage treatment will stimulate the muscle tone and muscle performance. It is even more important to the professional athlete who uses sports massage to enhance performance.
- A recently published study (Cezar et al., 2016) in the very prestigious scientific publication Proceedings of the National Academy of Sciences illustrates how mechanical stimulation of the injured skeletal muscle helps its regeneration.
The authors created local ischemia in the tibialis anterior muscle of rats and after complete loss of function was registered in the animals’ legs where mechanically stimulated by an implanted magnetic device (first group) and externally placed air cuff which compressed muscle in kneading type strokes (second group).
After two weeks of stimulation the authors registered a 2.5 fold increase in muscle regeneration and reduced fibrosis in the injured muscles compared to no-treatment controls. Fig. 6 illustrates the authors’ findings.
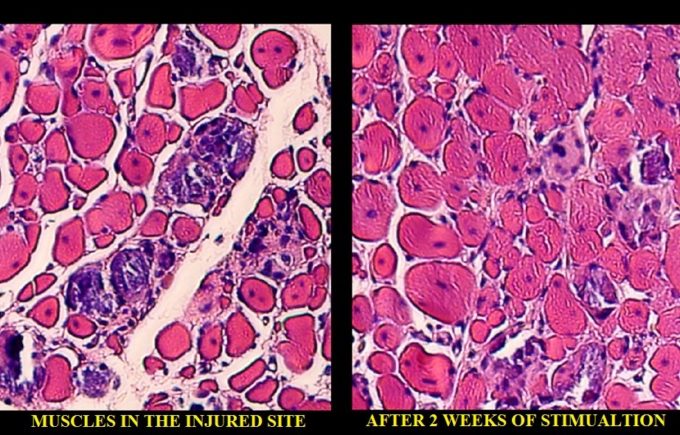
Fig. 6. Histological sample of the muscle after local ischemia and after 2 weeks of mechanical stimulation by kneading type strokes.
On the left you see histological changes in the tibialis anterior muscle after local ischemia was triggered there. The red colored areas are muscle cells and white colored areas are the fibrotic tissue developed between the muscle fibers as a result of local ischemia. At this point the animal completely lost function of the affected muscle. On the right, you see the same tissue after 2 weeks of mechanical stimulation with kneading type of compressions. The white areas of fibrosis are completely gone and normal structure of the muscle is completely restored. It coincided with complete restoration of lost function.
At the end of this review, I would like to quote the conclusion from an article by Maniotis, et al., (1997) published in Proceedings of the National Academy of Sciences of the USA:
“… direct mechanical linkages throughout living cells raise the possibility that regulatory information, in the form of mechanical stress or vibration (emphasized by R. T.), may be rapidly transferred from these cell surface receptors to distinct structures in the cell and nucleus, including ion channels, nuclear pores, nucleoli, chromosomes, and perhaps even individual genes, independent of ongoing chemical signaling mechanisms.”
REFERENCES
Brunett, D.M.: Mechanical Stretching Increases the Number of Epithelial Cell Synthesizing DNA in Culture. J. Cell Sci, 69: 34-35, 1984.
Cezar C.A., Roche E.T., Vandenburgh H.H., Duda G.N., Walsh C.J., Mooney D.J. Biologic-free mechanically induced muscle regeneration PNAS 2016 113 (6) 1534-1539
Chen, B-M., Grinnell, A.D.: Integrins and Modulation of Transmitter Release from Motor Nerve Terminals by Stretch. Science, 269: 1578-1580, 1995.
Curtis, A.S.G., Sheehar, G.M.: The Control of Cell Division by Tension or Diffusion. Nature, 274: 52-53, 1978.
Davidson, C.J., Ganion, L.R., Gehlsen, G.M., Verhoestra, B., Roepke, J .E., Sevier, T.I.: Rat Tendon Morphologic and Functional Changes Resulting from Soft Tissue Mobilization. Med. Sci. Sports Exer., 29: 313-319, 1997.
Gataulin, R.R., Zripov, A.T.: The Role of Cytoskeleton in Mechanoreceptor Activity of Pacinian Corpuscles. In: Mechanoreceptors: Development, Structure and Function: 209-211. Edited by E.P. Hnik, T. Soukup, R. Vejsada, J. Zelena. “Premium Press”, New York, 1988.
Gehlsen, G.M., Ganion, L.R., Helllfst, R.: Fibroblast Responses to Variation in Soft Tissue Mobilization Pressure. Med. Sci. Sports Med., 31(4): 531-535, 1999.
Geneser, F.: Texbook Of Histology. “Munksgaard Lea & Febiger”, Philadelphia, 1986.
Jain, M.K., Berg, R.A., Tandon, G.P.: Mechanical Stress and Cellular Metabolism in Living Soft Tissue Composites. Biomaterials, 11: 465-471, 1990.
Komuro, 1., Katoh, Y., Kaida, T., Shibazaki, Y., Kurabayashi, M., Hoh, E., Takaku, F., Yazaki, Y.: Mechanical Loading Stimulates Cell Hypertrophy and Specific Gene Expression in Cultured Rat Cardiac Myocytes. J. Biol. Chem., 266(2): 1265-1268.
Maniotis, A.J., Chen, C.s., Ingber, D.E.: Demonstration of Mechanical Connections Between lntegrins, Cytoskeletal Filaments, and Nucleoplasm that Stabilize Nuclear Structure. Proc. Natl. Acad. Sci. USA, 94(3): 849-854, 1997.
Shirinsky, V.P., Antonov, A.S., Birukov, K.G., Sobolevsky, A.V., Romanov, Y.A., Kabaeva, N.V., Antonova, G.N., Smirnov, V.N.: Mechano-Chemical Control of Human Endothelium Orientation and Size. J. Cell Biol., 109, 331-339, 1989.
Wang, N., Butler, J .P., Ingber, D.E.: Mechanotransduction Across the Cell Surface and Through the Cytoskeleton. Science, 260: 1124-1127, 1993.
Category: Medical Massage
Tags: 2017 Issue #2
